Abstract
Introduction: Relief of symptoms and splenomegaly is an important treatment goal for all patients with myelofibrosis (MF). In August 2019, fedratinib (FEDR) became the second treatment to be approved for intermediate- or high-risk primary or secondary MF. This study sought to describe clinical outcomes (spleen size, and hematologic and MF-specific symptoms) during the initial 6 months of FEDR therapy among real-world patients prescribed FEDR per label in the USA after prior treatment with ruxolitinib (RUX).
Methods: Patients with intermediate- or high-risk MF who initiated FEDR on or after August 16, 2019 (FEDR approval date) with ≥ 90 days of follow-up were identified from community oncology practices in the USA. The patients' treating physicians selected eligible patients and completed an electronic case report form. Data collection occurred in February and March 2021. Eligible patients were adults with MF treated with RUX and then FEDR, had spleen palpation conducted at initiation of FEDR, and had completed ≥ 1 cycle of FEDR. Providers abstracted patient clinical characteristics, dose of FEDR, spleen size, a symptom list derived from the Myelofibrosis Symptom Assessment Form version 4.0 (MFSAF v4.0), platelet count, hemoglobin (Hb), and white blood cell (WBC) count at each provider visit up to the first 6 months of FEDR therapy. Mean spleen size by month and best percentage change in spleen size over follow-up are described (eg, greatest numeric reduction in spleen size at any point compared with at initiation of FEDR). Mean spleen size, symptom count, platelet count, Hb, and WBC count at initiation of FEDR versus 3 months post FEDR, and versus 6 months post FEDR, were compared via paired 2-sided t-test. Proportions (ie, percentage reduction in spleen size and proportion of transfusion-dependent patients) between initiation of FEDR versus 3 and 6 months, respectively, were compared via 1-sided chi-square test. Only patients assessed at initiation (ie, with palpable spleen or complete blood counts available) and with a corresponding assessment at 3 months or 6 months were included in the comparative analysis. P value < 0.05 was considered statistically significant.
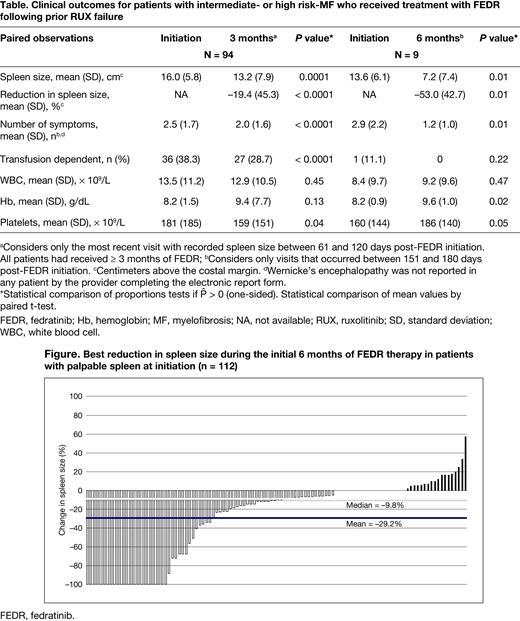
Results: Data for 150 eligible patients were collected and analyzed. Mean age at time of FEDR initiation was 68 (range, 36-85) years. Median duration of RUX prior to FEDR was 7.6 (range, 0.3-65.5) months; 50% received RUX at a dose ≥ 20 mg twice a day. At FEDR initiation, 43% of patients had International Prognostic Scoring System (IPSS)/Dynamic IPSS High-risk disease, 37% Intermediate (Int)-2 risk, 9% Int-1 risk, and 10% unknown. FEDR was started at 400 mg once a day in 74% of patients. Median time of follow-up post-FEDR initiation was 5 (range, 3-17) months. The median duration of FEDR was 4.4 (range, 3.5-7.0) months. At data cutoff, 55% of patients were still on FEDR. Rationale for discontinuation of FEDR included disease progression (43%), patient choice (19%), allogeneic stem cell transplant (18%), toxicity (6%), persistent symptoms (5%), and other (9%); 44 patients died after discontinuation. The median duration of treatment for patients still on FEDR at data cutoff was 4.9 (range, 3.0-17.3) months versus 4.0 (range, 3.0-10.6) months for patients who had discontinued FEDR prior to data cutoff. At initiation of FEDR, 88% of patients had palpable spleen: mean spleen size was 16.0 (standard deviation [SD], 5.8) cm, which declined to 13.2 (SD, 7.9) cm at 3 months (P = 0.0001) and 7.2 (SD, 7.4) cm at 6 months (P = 0.01) (Table). Mean number of symptoms declined significantly, and mean platelet count increased significantly at 3 and 6 months (Table). Mean best percentage change in spleen size was −29.2% (median, −9.8%) (Figure); complete resolution of palpable spleen occurred in 21% of patients.
Conclusions: This study provides some of the first real-world evidence of the effectiveness of FEDR when used post RUX failure in patients with intermediate- or high-risk MF. In this study, FEDR provided statistically significant reductions in spleen size and reported number of MF-related symptoms. A longer duration of FEDR led to greater clinical benefits in relation to these parameters, illustrating the real-world benefit of FEDR when utilized post RUX discontinuation.
Mascarenhas: Merus: Research Funding; CTI Biopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Geron: Consultancy, Research Funding; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Forbius: Research Funding; Prelude: Consultancy; Promedior: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Galecto: Consultancy; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Gerds: PharmaEssentia Corporation: Consultancy; Novartis: Consultancy; Sierra Oncology: Consultancy; AbbVie: Consultancy; Celgene/Bristol Myers Squibb: Consultancy; Constellation: Consultancy; CTI BioPharma: Research Funding. Kee: Bristol Myers Squibb: Current Employment. Kish: Cardinal Health: Current Employment, Current equity holder in publicly-traded company, Research Funding. Abraham: Bristol Myers Squibb: Current Employment. Balanean: Cardinal Health: Current Employment; Georgia State University: Other: former student and employee. Nadal: Bristol Myers Squibb: Current Employment. Liassou: Cardinal Health: Current Employment. Feinberg: Cardinal Health: Current Employment. McBride: BMS: Current Employment. Harrison: Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sierra Oncology: Honoraria; Incyte Corporation: Speakers Bureau; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Galacteo: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Keros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Constellation Pharmaceuticals: Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Promedior: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.